Dietary retinyl esters remodeling during digestion
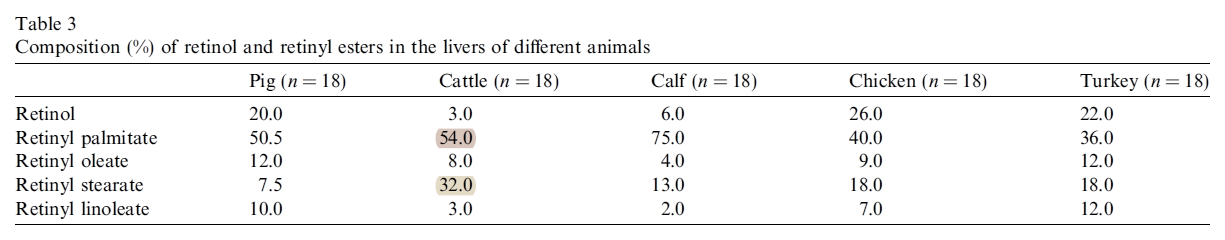
I didn’t recall that cattle liver had a substantial portion of poisonol esterified with stearate [1]Majchrzak, Dorota, Elisabeth Fabian, and Ibrahim Elmadfa. “Vitamin A content (retinol and retinyl esters) in livers of different animals.” Food Chemistry 98.4 (2006): 704-710. https://doi.org/10.1016/j.foodchem.2005.06.035:
The original profile can be lost during digestion [2]Ross, A. Catharine. “Vitamin A: physiology, dietary sources, and requirements.” Encyclopedia of Human Nutrition. Elsevier Inc., 2012. 333-339. https://doi.org/10.1016/B978-0-12-375083-9.00273-7:
Dietary retinyl esters must be hydrolyzed in the lumen of the small intestine, emulsified, and incorporated into lipid micelles before retinol can be absorbed into the mucosa
(Figure 3). Several retinyl ester hydrolase (REH) enzymes are present in pancreatic juice or situated on the brush border of duodenal and jejunal enterocytes. For greatest efficiency, these processes require an adequate amount of bile salts and a small quantity of dietary fat, approximately 5%, consumed concomitantly. The retinol molecules then diffuse into the enterocyte where they are bound to CRBP-II and then esterified by the enzyme lecithin:retinol acyltransferase (LRAT). Animals lacking LRAT have been shown to absorb vitamin A poorly, consistent with observations that retinyl ester formation is important for vitamin A absorption.
All experiments below employed doses that are equivalent to about 15 mg of poisonol, which is easy to obtain with supplements.
Minimal liberation occurs at the stomach level, it’s mostly when the meal reaches the intestine [3]Borel, Patrick, et al. “Processing of vitamin A and E in the human gastrointestinal tract.” American Journal of Physiology-Gastrointestinal and Liver Physiology 280.1 (2001): G95-G103. https://doi.org/10.1152/ajpgi.2001.280.1.G95:
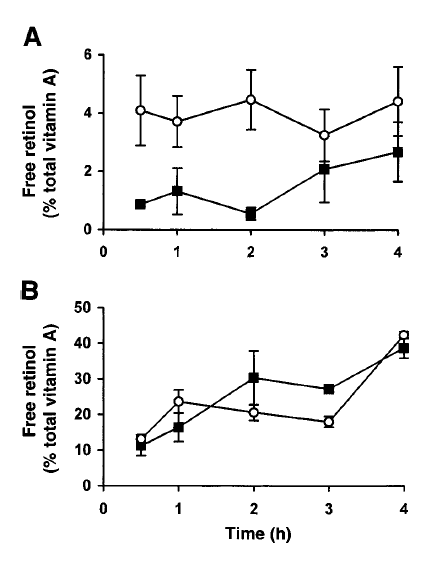
A was retinyl palmitate and 0.3% was free retinol. Results were obtained after ingestion of the fine emulsion (○) and after ingestion of the coarse emulsion (■). Values are mean ± SE of 8 measurements.
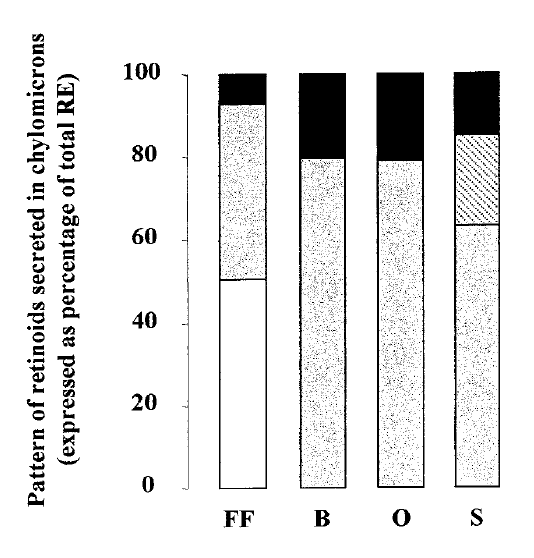
The toxin dose, quantity and quality of fat can influence the esters pattern as well. It’s most likely to reflect the profile of the fat consumed when the dose of poison and fat are high in conjuction [4]Borel, Patrick, et al. “Dietary triglycerides, up to 40 g/meal, do not affect preformed vitamin A bioavailability in humans.” European journal of clinical nutrition 51.11 (1997): 717-722. https://doi.org/10.1038/sj.ejcn.1600466:
[..]two pathways coexist for the esterification of retinol in the enterocyte. In the first one, LRAT (Quick & Ong, 1990) esterifies CRBP-II bound retinol (Levin, 1993) with palmitate and stearate found in the sn-1 position of microsomal phosphatidylcholines. In the second one, ARAT esterifies free retinol with acyl-CoA (Rasmussen et al, 1984) produced in vivo. Since in this study dietary TG were provided as sunflower oil rich in linoleic acid (65±70% of fatty acids), it is likely that retinyl linoleate found in the CMs originated from linoleyl-CoA and thus, reflected the activity of ARAT. The result obtained, i.e. the stepwise increase in the proportion of retinyl linoleate, among other retinyl esters, when the amount of meal TG increased, suggests the following mechanism for retinol esterification in the human enterocyte: when very few amount of dietary TG is available, acyl-CoA concentration in the enterocyte is very low and retinol is almost exclusively (98.5%) esterified by LRAT (1.5% retinyl linoleate recovered after the intake of the meal with no added fat), but when a higher amount of dietary TG is available, then the relative amount of acyl-CoA in intracellular pool increases and ARAT esterify a significant fraction (up to 9.5%) of absorbed retinol. Thus, LRAT is the main retinol esterifying enzyme in humans, but ARAT can esterify a fraction of retinol when pharmacological doses of retinol are provided along with common quantities of dietary TG in line with data obtained in cynomolgus monkeys (Collins et al, 1992).
Fat in the meal is desirable not only for enhancing absorption, but also because it can prevent free poisonol from appearing if the esterification capacity is overwhelmed [5]Sauvant, Patrick, et al. “Amounts and types of fatty acids in meals affect the pattern of retinoids secreted in human chylomicrons after a high-dose preformed vitamin A intake.” Metabolism 52.4 (2003): 514-519. https://doi.org/10.1053/meta.2003.50082: